Abstract
Background: Pts with HR-MDS unfit for hematopoietic stem cell transplant (HSCT) have poor outcomes and limited survival with single-agent hypomethylating agent (HMA) therapy. Novel treatments that provide durable responses and clinically meaningful survival benefit for pts with HR-MDS are needed. TIM-3 is a promising target in myeloid malignancies as it regulates innate and adaptive immune cells and is expressed on leukemic stem cells (LSCs) and blasts but not normal hematopoietic stem cells. Sabatolimab (MBG453) is a novel immuno-myeloid therapy that binds to TIM-3 on immune cells, facilitating antileukemic immune activation and phagocytic killing of leukemic cells. Sabatolimab also binds to TIM-3 on leukemic cells, potentially impeding self-renewal of LSCs via inhibition of TIM3-galectin-9. In an early phase (ph) trial (NCT03066648) sabatolimab + HMA therapy showed a durable clinical benefit and was well tolerated in pts with HR-MDS (Wei, EHA 2021).
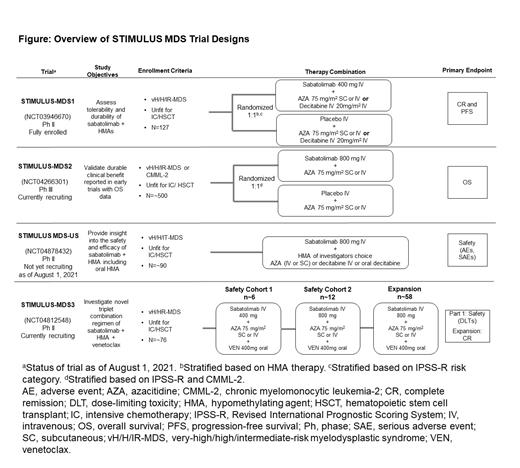
Study Design and Methods: The STIMULUS immuno-myeloid clinical trial program is investigating the safety, efficacy, and durability of multiple sabatolimab-based combination therapies in pts with myeloid malignancies. Additional exploration of the mechanism of action of sabatolimab and identification of potential biomarkers predictive of response is planned. This abstract summarizes the design of the 4 ongoing STIMULUS trials in previously untreated adult pts with HR-MDS who are not eligible for intensive chemotherapy (IC) or HSCT.
STIMULUS-MDS1 (NCT03946670) is an international, randomized, double-blind, placebo-controlled Ph II trial evaluating sabatolimab + HMA therapy in pts with very-high, high-, or intermediate-risk (vH/H/IR)-MDS which has completed enrollment (N=127). The primary endpoints of STIMULUS-MDS1 are complete response (CR) and progression-free survival (PFS).
STIMULUS-MDS2 (NCT04266301) is an ongoing, international, randomized, double-blind, placebo-controlled, Ph III trial of sabatolimab + azacitidine (AZA) in pts with vH/H/IR-MDS or chronic myelomonocytic leukemia-2. Target enrollment is ~500 pts. The primary endpoint in this trial is overall survival (OS). Key secondary endpoints are time to definitive deterioration of fatigue; duration of RBC transfusion-free interval; and improvement of fatigue, physical functioning, and emotional functioning. Other endpoints include CR, marrow CR (mCR), partial remission (PR), PFS, and leukemia-free survival (LFS). STIMULUS-MDS2 is a trial that aims to determine if sabatolimab + HMA has a statistically significant and clinically meaningful benefit in OS and quality of life vs HMA alone.
STIMULUS MDS-US (NCT04878432) is a US-based, open-label, single-arm, Ph II trial investigating sabatolimab + HMAs of investigators' choice (AZA intravenous [IV] or subcutaneous, decitabine IV or oral decitabine) in pts with vH/H/IR-MDS. Target enrollment is 90 pts. Primary endpoint is safety, which will be evaluated via incidence and severity of adverse events (AEs) and serious AEs. Secondary endpoints include CR, PFS, LFS, and OS. This trial will provide further understanding of sabatolimab + HMA therapy with additional insight into the safety and efficacy of sabatolimab + oral HMA.
STIMULUS-MDS3 (NCT04812548) is an international, open-label, single-arm, Ph II trial that explores triplet therapy of sabatolimab combined with AZA and the BCL-2 inhibitor venetoclax (VEN) in pts with vH/HR-MDS. Part 1 of this trial is a safety run-in comprising 2 safety cohorts with ~6 pts receiving sabatolimab 400 mg + AZA + VEN and ~12 pts receiving sabatolimab 800 mg + AZA + VEN. Primary endpoint of part 1 is safety (dose-limiting toxicities between cycle 1 day 8 and end of cycle 2). If (both 400 and 800 mg) sabatolimab + AZA and VEN are safe, the trial will progress to an expansion cohort (~58 pts) of sabatolimab 800 mg Q4W + AZA + VEN. The primary endpoint is CR. Secondary endpoints include CR + mCR rate, overall response rate (CR + mCR + PR + hematologic improvement), OS, PFS, LFS, and event-free survival.
The STIMULUS immuno-myeloid clinical trial program is investigating the efficacy, safety, and improved quality of life of multiple sabatolimab-based combination therapies in pts with myeloid malignancies. The goal of ongoing STIMULUS-MDS trials is to gain insight into the promising durable benefit of sabatolimab combination therapies in pts with HR-MDS.
Zeidan: Jazz: Consultancy; Jasper: Consultancy; Pfizer: Other: Travel support, Research Funding; AstraZeneca: Consultancy; Janssen: Consultancy; Novartis: Consultancy, Other: Clinical Trial Committees, Travel support, Research Funding; Loxo Oncology: Consultancy, Other: Clinical Trial Committees; Ionis: Consultancy; Kura: Consultancy, Other: Clinical Trial Committees; Incyte: Consultancy, Research Funding; Gilead: Consultancy, Other: Clinical Trial Committees; Genentech: Consultancy; Epizyme: Consultancy; Daiichi Sankyo: Consultancy; Geron: Other: Clinical Trial Committees; BMS: Consultancy, Other: Clinical Trial Committees, Research Funding; Cardiff Oncology: Consultancy, Other: Travel support, Research Funding; Boehringer Ingelheim: Consultancy, Research Funding; BioCryst: Other: Clinical Trial Committees; BeyondSpring: Consultancy; Astex: Research Funding; Astellas: Consultancy; Aprea: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Agios: Consultancy; ADC Therapeutics: Research Funding; Acceleron: Consultancy, Research Funding; AbbVie: Consultancy, Other: Clinical Trial Committees, Research Funding. Al-Kali: Astex: Other: Research support to institution; Novartis: Research Funding. Borate: Takeda: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees, Other: Advisory Board; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Jazz Pharma: Research Funding; Blueprint Medicine: Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Membership on an entity's Board of Directors or advisory committees; incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding; Rampal: Membership on an entity's Board of Directors or advisory committees; Galecto, Inc.: Consultancy; Promedior: Consultancy. Cluzeau: Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel, accommodations, expenses, Speakers Bureau; Abbvie: Consultancy, Honoraria, Speakers Bureau; Agios: Honoraria; Amgen: Speakers Bureau; Jazz Pharma: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Astellas: Speakers Bureau; BMS/Celgene: Consultancy, Honoraria, Speakers Bureau; Pfizer: Other: travel, accommodations, expenses; Takeda: Other: travel, accommodations, expenses. DeZern: Taiho: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees. Esteve: Jazz: Consultancy; Novartis: Consultancy, Research Funding; Astellas: Consultancy; Abbvie: Consultancy; Bristol Myers Squibb/Celgene: Consultancy; Pfizer: Consultancy; Novartis: Research Funding. Giagounidis: Novartis: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees. Platzbecker: Celgene/BMS: Honoraria; Novartis: Honoraria; Janssen: Honoraria; Geron: Honoraria; AbbVie: Honoraria; Takeda: Honoraria. Sallman: Kite: Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Agios: Membership on an entity's Board of Directors or advisory committees; Incyte: Speakers Bureau; Syndax: Membership on an entity's Board of Directors or advisory committees; Intellia: Membership on an entity's Board of Directors or advisory committees; Shattuck Labs: Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy; Aprea: Membership on an entity's Board of Directors or advisory committees, Research Funding; Magenta: Consultancy. Santini: Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Menarini: Membership on an entity's Board of Directors or advisory committees; BMS/Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Geron: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Astex: Membership on an entity's Board of Directors or advisory committees. Sanz: Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, and expenses, Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, and expenses, Speakers Bureau; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, and expenses; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Helsinn Healthcare: Consultancy, Membership on an entity's Board of Directors or advisory committees; Boehringer Ingelheim: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead Sciences: Other: Travel, accommodations, and expenses. Sekeres: Novartis: Membership on an entity's Board of Directors or advisory committees; Takeda/Millenium: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees. Wei: Astra Zeneca: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene/BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Agios: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Abbvie: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Astellas: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Servier: Membership on an entity's Board of Directors or advisory committees, Research Funding; Macrogenics: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees. Van Hoef: Novartis Pharma: Current Employment. Nourry-Boulot: Novartis: Current Employment. Sadek: Novartis: Current Employment. Bengoudifa: Novartis Pharma AG: Current Employment. Sachs: Novartis Pharma AG: Current Employment. Sabo: Novartis: Current Employment, Current holder of stock options in a privately-held company.
Sabatolimab is a novel immuno-myeloid therapy targeting TIM-3 and is under investigation for the treatment of patients with myeloid malignancies